In the context of the implementation of the National Vaccination Plan for COVID-19, the Vaccination Portal opens on Tuesday, March 9, at 8 a.m., with 22,808 appointments for Vaccination Centers operating in all Provinces. They will have priority for arranging appointments people aged 67 and over.
Until Sunday, March 7, 99,275 vaccinations were performed, of which 70,534 are vaccinations with 1η dose and 28,741 relate to individuals who received both doses and completed their vaccination. Simultaneously, 1ηThe vaccine dose was given to 1,277 people in vulnerable groups. The rate of vaccination of individuals at increased risk of serious disease from COVID-19 increases in the following days, aiming to cover at least 1η dose, about 30,000 people by the end of the month.
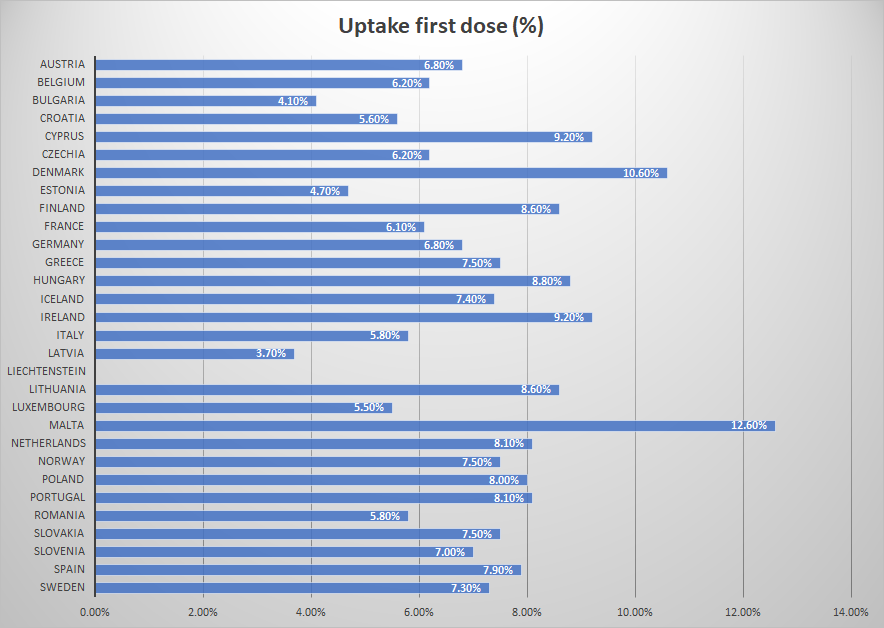
According to the data of the European Center for Disease Control and Prevention (ECDC), Cyprus has been vaccinated with 1η dose 9,2% of the population 18 years and older and is in 3η position of European countries on vaccination coverage.
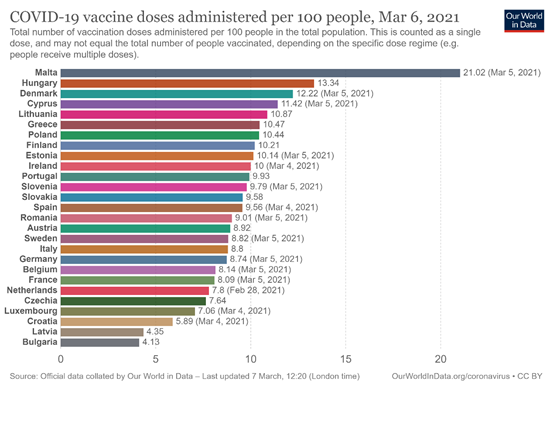
At the same time, according to the data published by the website OurWorldInData, Cyprus remains in the first places among the countries of the European Union in terms of the administration of at least one dose of vaccines, with an average of 11,42 doses per 100 people (data until March 5 ). At the same time, Cyprus has completed to a very high degree its main goal of ensuring the protection, through vaccination, of the elderly over 75 years of age, who have an increased chance of serious illness and death. At the same time, following the ECDC recommendations, Cyprus has reached the European Union target of vaccinating at least 80% of health professionals by March.
Cyprus continues vaccinations on the basis of the available licensed vaccines received on a weekly basis from Pfizer / BioNTech, Moderna and AstraZeneca. It is expected that within a week the European Medicines Agency will evaluate the application of Johnson & Johnson for the licensing of its vaccine, which is a single dose.