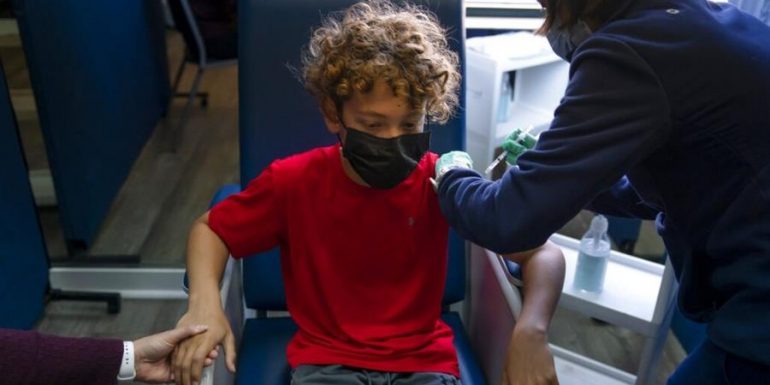
Clinical trials in children aged five to eleven have been started by Pfizer and Moderna, according to a New York Times article.
According to the American newspaper, this is an initiative that comes at the request of the US Food and Drug Administration (FDA).
Encouragement for clinical trials in this age group is done to help identify cases of rare heart disease that have occurred in people under the age of 30 who have been fully vaccinated (have done both doses of the Covid disease vaccine).
In a statement on the CNET website, Pfizer acknowledged that it had begun clinical trials in children aged 5 to 11 on June 8 and in children under 5 on June 21.
The study included up to 4.500 participants from the United States, Finland, Poland and Spain, according to the US pharmaceutical giant.
Initial results for the age group 5 to 11 years are expected in September and data for children aged 2 to 5 years, as well as 6 months to 2 years, by the end of the year.
Moderna began recruiting volunteers in this age group last March and confirmed to CNET that it is starting clinical trials in children aged five to 11.
It is recalled that, last week, the European Medicines Agency (EMA) approved the Moderna vaccine against COVID for children aged 12 to 17 years.
When the studies began, the two companies Pfizer and Moderna included three thousand children, aged 5 to 11 years.
However, companies are now being asked to double the number of participants, according to the controversial publication of the American newspaper.
Source: Protothema.gr