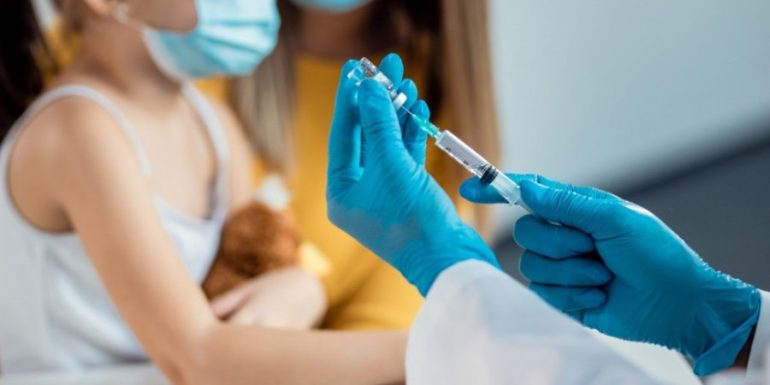
Partners Pfizer (USA) and BioNTech (Germany) have announced that they will test a third dose of 3 μg (micrograms) of their coronavirus mRNA vaccine in children under five years of age.
The two doses of the vaccine, which were tested in two-, three- and four-year-olds, failed to elicit an immune response comparable to that in adolescents and adults. On the other hand, their vaccine gave birth to an immune response in children six months to two years old.
The companies assured that "no safety issues were detected" and stated that "the decision to evaluate a third installment in children aged six months to five years reflects the companies' commitment to carefully choose the right dose to optimize the risk-benefit profile". .
The "blind" clinical trial will now include a third dose at least two months after the second dose, with the aim of increasing the protection of children up to five years of age. If the three installments are finally successful, the companies have announced that they will submit their test data to regulators within the first half of 2022, so that they can receive an urgent marketing authorization for their vaccine in young children.
They also announced that they plan to try a third dose of 10 μg in children five to 12 years old. They also started a third dose subset of 10 or 30 μg in 600 adolescents aged 12-17 years. These tests are related to the emergence of the new Omicron variant and the initial findings that people who take a booster dose have a greater degree of protection.